

On slow heating in an open container, so that the water vapor pressure over the solid is practically zero, water evaporates out of each of the solid 6-, 2-, and 1- hydrates, leaving the next lower hydrate, at about 40☌, 89☌, and 125☌, respectively. On rapid heating or in a closed container, each of the 6-, 2-, and 1- hydrates partially melts into a mixture of the next lower hydrate and a saturated solution-at 51.25 ☌, 206 ☌, and 335 ☌, respectively.

The anhydrous compound can be prepared by heating the hydrates. The monohydrate and the anhydrous forms can be obtained by cooling solutions only under high pressure, above 206 ☌ and 335 ☌, respectively. Water ice, rather than cobalt chloride, will crystallize from solutions with concentration below 29%. Cooling saturated aqueous solutions yields the dihydrate between 120.2 ☌ and 51.25 ☌, and the hexahydrate below 51.25 ☌. The solid dihydrate and hexahydrate can be obtained by evaporation. Preparation Ĭobalt chloride can be prepared in aqueous solution from cobalt(II) hydroxide or cobalt(II) carbonate and hydrochloric acid: The octahedron is completed by a pair of mutually trans aquo ligands. Each Co center is coordinated to four doubly bridging chloride ligands. The dihydrate, CoCl 2(H 2O) 2, is a coordination polymer. The anhydrous salt is hygroscopic and the hexahydrate is deliquescent. This species dissolves readily in water and alcohol. The crystal unit of the solid hexahydrate CoClĢO contains the neutral molecule trans- CoClĤ and two molecules of water of crystallization. Concentrated solutions are red at room temperature but become blue at higher temperatures. Under atmospheric pressure, the mass concentration of a saturated solution of CoClĢ in water is about 54% at the boiling point, 120.2 ☌ 48% at 51.25 ☌ 35% at 25 ☌ 33% at 0 ☌ and 29% at −27.8 ☌. Solutions Ĭobalt chloride is fairly soluble in water.

The vapor pressure has been reported as 7.6 mmHg at the melting point. At about 706 ☌ (20 degrees below the melting point), the coordination is believed to change to tetrahedral. With a decrease in moisture, the colors change in the reverse order to that given above, and the blue color returns when the air becomes dry.At room temperature, anhydrous cobalt chloride has the cadmium chloride structure ( CdClĢ) (R 3m) in which the cobalt(II) ions are octahedrally coordinated. As the moisture in the atmosphere increases, the color changes first to bluish red, then light red and finally pink, according to the amount of moisture.
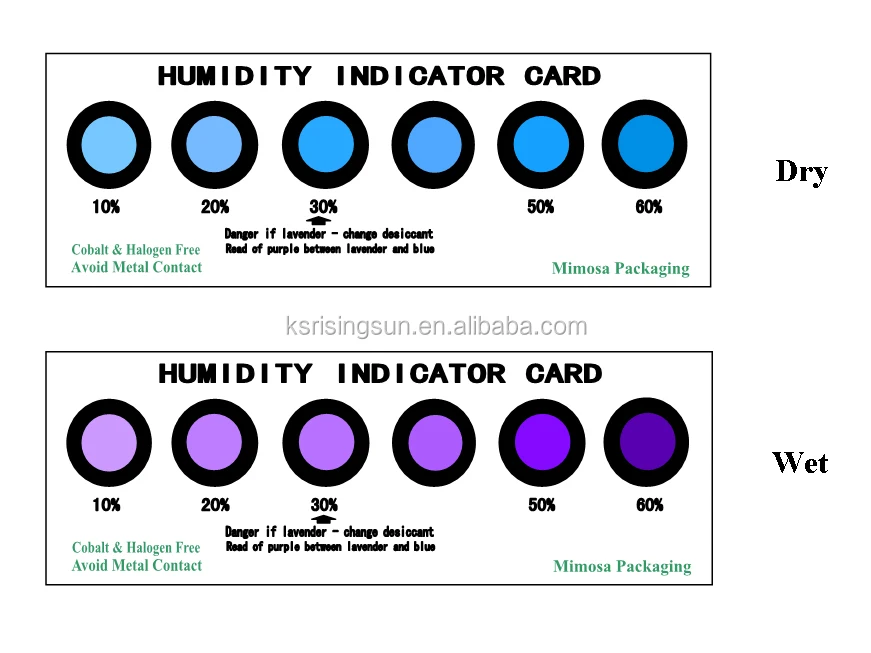
This cloth will change color as the amount of moisture in the atmosphere changes, the change being due to the cobalt salt, which, in dry air, is lavender blue. A simple weather indicator that may be used in determining the condition of the atmosphere may be made as follows: Dress a small figure, in the form of a doll, with a piece of cloth, previously dipped in the following solution: Chloride of cobalt,ģ0 parts by weight sodium chloride, 15 parts gum arabic, 7 1/2 parts calcium chloride, 4 1/2 parts, and water, 400 parts.


 0 kommentar(er)
0 kommentar(er)
